Alcoholic beverages
Many people consume alcohol, some at parties, others during meals or at some type of celebration. This is done because of the ignorance, many of the alcohol consumers do not know where the drink they are ingesting comes from, much less how it affects their body. The form of production, the degree of toxicity and the alcoholic graduation of the most consumed and harmful beverages in general will be evaluated.
TOXICITY OF ALCOHOLS
Ingestion of large quantities of ethyl alcohol causes intoxication, abuse of these beverages can lead to chronic alcoholism, toxocomania or acute alcoholism. The human organism is capable of oxidizing and eliminating in the form of water a maximum of 0.8 cubic centimeters per hour and per kilogram of body weight. Beyond that amount, the ingested alcohol goes to the blood and nervous tissues, causing toxic effects.
Regardless of whether it is a drug capable of generating dependency, alcohol consumption has been convincingly associated with about 60 different types of diseases and undesirable circumstances, including mental and behavioral disorders, gastrointestinal conditions, cancers, diseases cardiovascular diseases, immune disorders, lung diseases, bone and muscle diseases, reproductive disorders and perinatal damage, including an increased risk of premature births.
ALCOHOLIC GRADUATION AND FORM OF PRODUCTION
The alcoholic strength or alcoholic strength of an alcoholic beverage is the expression in degrees of the number of volumes of alcohol (ethanol) contained in 100 volumes of the product, measured at a temperature of 20 ° C. It is a measure of percentage concentration in volume.
The different drinks are differentiated by their alcoholic graduation, not by the type of alcohol, which is always the same; depending on the type of processing is distinguished between:
- Fermented beverages: its graduation is between 3.5 and 15 degrees. (eg).
- Beer, whose alcohol content is between 4 ° and 5 ° .The type of alcoholic fermentation of beer is where the action of the cimasa secreted by the yeast converts simple sugars, such as glucose and fructose, into ethyl alcohol and carbon dioxide.
- Wine, which has an alcohol content between 11 ° and 14 °. In the case of wines, the chemistry of fermentation is the derivation of carbon dioxide from the air that penetrates the leaves of the vineyard and then is converted into starches and their derivatives . During absorption in the grapes, these bodies are converted into glucose and fructose (sugars). During the fermentation process, the sugars are converted into ethyl alcohol and carbon dioxide according to the formula C6H12O6 -> 2C2H5OH + 2CO2.
- Cider is a low-grade alcoholic beverage (from 2% by vol. To a maximum of 8% by vol.). Cider fermentation is based on a succession of biochemical transformations of the components of apple must and the products resulting from these, carried out by yeasts, lactic bacteria and acetic bacteria. The most relevant fermentation is the alcoholic fermentation transforming the sugar into alcohol. The second is the so-called malolactic that produces important sensory changes in the cider, when taken to a notable loss of acidity and an increase in finished volatile components, mainly acid esters and alcohols. In addition, this biochemical process promotes greater microbiological stability.
- Vodka (GA 40 °) Originally the production of this drink was from the cheapest and most abundant local agricultural products such as wheat, corn, potatoes, sugar cane or the combination of any of these. The process consisted of a simple and rapid filtration of the yeast from these vegetables using a charcoal-based filter, rather than an expensive and prolonged distillation process. The purified liquid was then reduced, without aging, until it was potabilized by the addition of distilled water and then bottled.
- Tequila (GA 60 °) The juice of Mezcal is collected in the preparation vats of musts, here is added the yeast (microorganism responsible for the fermentation process) already adapted to the medium from a previous day. Once prepared, the musts are pumped to the fermentation room, where they remain for about 72 hours. (this is done in volumes of 30,000 lts). Here the fermentation chemical reaction is carried out, ie the sugars will be converted into ethyl alcohol.
- Ron (GA 40 °) A must is first prepared with molasses, distillation residues (vinasse), sometimes also with foams from defecation of cane juice, and water. A fermentation process is then carried out on the must with various species of yeast, which may be favored by inoculation. After a period of time comprising from 2 to 5 days, the fermentation ends and the alcoholic liquor obtained is distilled at 55-65 degrees in a still or distillation column. The resulting spirit (average distillation fraction) is allowed to age in oak barrels.
- Whiskey (GA 40 °) The first stage of its production consists in grinding the grains, then adding water to the prepared and cooked grind to obtain a sugar solution called wort. This is filtered and poured into fermentation vessels, and yeast is added. The yeast converts the sugars present in this substance into ethyl alcohol and carbon dioxide. Once the fermentation is completed, the malt or low-grade beer obtained is normally distilled using one of two methods: continuous distillation or two or more distillations by alembic lots (used especially in Scottish malt whiskey distilleries). distillation the produced liquor is aged in wooden vats.
But if the distilled beverages are also fermented, then what is the difference?
That once the fermentation is finished, instead of directing the production process for its consumption, taking advantage of all its properties and nutrients, these begin another stage of production: distillation.
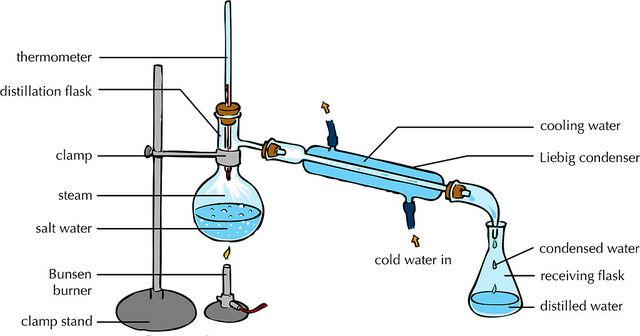
Distillation in its most traditional form involves heating the fermented liquid into a closed vessel until the alcohol (with a boiling point less than that of the water) evaporates. Alcohol droplets will be concentrated to the center of the container roof and condensed by draining through a tube into another container. That way, the new vessel will contain higher purity alcohol, leaving behind water and other compounds. As more distillation advances, more water will begin to pass, reducing the percentage of alcohol.
Usually the distillates carry a second or a third distillation, repeating this process. That way you make sure to have more pure alcohols, but ...
ALCOHOLEMIA TEST
Alcohol test or BAC test measures blood alcohol concentration. It is obtained by means of a percentage of mass, mass by volume or a combination. For example, a level of 0.3 alcohol in blood means 0.3 g of alcohol per liter of blood. When measured by the alcohol detected in the exhaled air, the unit used is "milligrams per liter of air", which in conventional practice is conventionally converted into "grams per liter of blood", multiplying by the coefficient 2.



.jpg)






Comentarios
Publicar un comentario